Technology
A broad and young IP portfolio protects the technology platform with 16 granted patent families, actively managed by a leading European patent and trade-mark attorney with a renowned international reputation.
Over 25 peer reviewed scientific articles provide a solid validation of the technology.
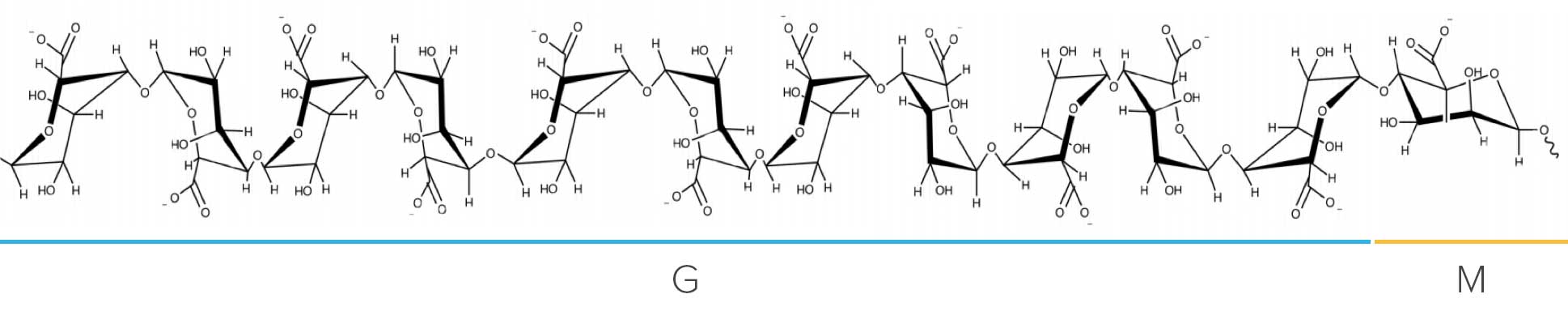
Representative structure of OligoG showing the composition of α-L-guluronic acid (G) and β-D-mannuronic acid (M) residues.
AlgiPharma’s OligoG technology is based on highly purified and defined oligosaccharides processed and isolated from the alginate polysaccharide extracted from the brown seaweed Laminaria hyperborea and manufactured according to GMP guidelines, ICH Q7 and ISO standards. Large scale land-based fermentation manufacturing processes are also in development.
AlgiPharma’s alginate oligosaccharides have been shown to:
- Release stagnant mucus in the airway and intestinal mucosa
- Restore intestinal transit times
- Enhance the delivery of drugs and lipid-based nanoparticle (LNP) therapeutics across mucus barriers
- Disrupt established microbial biofilms in airways and infected wounds
- Prevent new biofilm formation and inhibit microbial adherence to surfaces
- Reduce microbial virulence factors
- Inhibit fungal hyphae formation and prevent tissue invasion of fungal cells
- Potentiate anti-bacterial and anti-fungal compounds
Our research also shows that alginate oligosaccharides do not induce microbial resistance.
This unique active ingredient is highly water-soluble, with a negative charge density and an affinity for multivalent cations. The molecule is stable, non-toxic and bio-compatible.
AlgiPharma’s alginate oligosaccharides can add functionality when conjugated with antimicrobials, reducing toxicity, and maintaining efficacy compared to the parent compound.